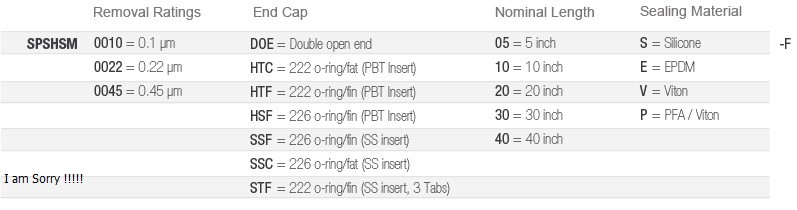
Raliable Microbiological Control
The primary purpose of a membrane filter cartridge in beverage processing is to effectively
control spoilage microorganisms.
| Typical Log Reduction Value(LRV) |
| B.diminuta | Lactobaccilus
Brevis | Sasharomyces
Cerevisiae |
| 0.1μm | >7/cm2 | N/A | N/A |
| 0.2μm | >7/cm2 | N/A | N/A |
| 0.45μm | N/A | >7/cm2 | N/A |
| 0.65μm | N/A | >4/cm2 | >7/cm2 |
| 1.2μm | N/A | N/A | >7/cm2 |
Log Reduction Values are calculated using the following formula:
LRV = log 10( total number of organisms entering the filter total number of organisms entering the filter)
Integrity Testing Parameters
The Integrity test is a non-destructive test that can be performed
by the user to ensure the filter is installed correctly and ready for operation.
| Bubble Point |
0.1μm
≥0.38MPa | 0.2μm
≥0.32MPa | 0.45μm
≥0.2MPa |
* 0.45μm (Diffusional Flow ≤25ml/min@1500mbar)